Petrology of Silicic Glasses in the Southwest |
M. Steven Shackley
University of California at Berkeley
![]()
The formation of silicic glasses (obsidian) is essentially a rare event in nature since a number of limiting factors must occur in order to form a glass. A brief discussion of rhyolite equilibrium melt reactions will be useful for the understanding of the occurrence of obsidian in the Southwest.
Obsidian is a glass, or a super-cooled liquid that is liquid in all its properties except in its ability to flow easily (Cann 1983). As a glass, its atomic structure, by definition, is entirely disordered. Because of this it has no preferred direction of fracture and is entirely isotropic, at least when entirely aphyric. This property endows obsidian with its excellent flaking properties and extremely sharp edges when fractured.
To a USGS photo glossary of volcanic rock terms
![]()
Trace Element Chemistry and Silicic Melts
Characterization of obsidian sources to determine the source is a function of the specific properties of silicic melts (Cann 1983). Obsidian lava is formed at depth during high temperature (ca. 1000º C) melt reactions (Cox et al. 1979). These melts are thus formed in equilibrium with the solid material and the trace elements present are distributed between the liquid and solids. During this process some elements such as chromium (Cr), cobalt (Co), and nickel (Ni) are strongly absorbed into the solids. These, and others, are called compatible elements because they are compatible with the crystallizing solids. Others (gallium and germanium) are evenly distributed between the solid and liquid phases. Still others, however, are called incompatible because they are incompatible with the solid phases and are concentrated in the liquid. This incompatibility may be due to the ions being too large for the available ionic sites in the liquid, such as with rubidium (Rb), cesium (Cs), strontium (Sr), and barium (Ba). Or, it may be due to the ions possessing too high a charge to fit the crystal structure of the solid phase such as the triply charged rare earth elements lanthanum (La), cerium (Ce), and yttrium (Y), the quadruply charged ions of titanium (Ti), and zirconium (Zr), or the quintuply charged ions of phosphorus (P), tantalum (Ta), and niobium (Nb).
As a magma evolves, further melting and crystallization change the nature of the solids, and crystals may form in the liquid which accept the incompatible elements. Some feldspars, for instance, are good hosts for strontium, as is mica for rubidium. "Evolved" obsidian magmas may contain these crystal "hosts" and the ratio of a given element between the liquid and solid phases will change dramatically. Changes of this kind issue a particular chemical character to a given obsidian. In peralkaline glasses, for instance, zirconium can be quite high (>1000 ppm), while in peraluminous glasses zircon or other zirconium bearing solids begin at much lower levels (Cann 1983). In peralkaline melts, barium and strontium are rapidly depleted by feldspars, while zirconium and niobium continue to be enriched to relatively high levels. This seems to have been the case with the San Francisco Peak glass where the formation of zircons absorbed the zirconium and feldspars absorbed the strontium (Burton 1986).
The result of these processes is that the incompatible element mix of a given obsidian source varies from any other and becomes a sensitive indicator of origin. It is even possible that in a single magma chamber, as the magma evolves, glass erupted at different times will have differing trace element chemistries. This seems to be the case at Sauceda Mountains where two chemical modes of Tertiary marekanites are mixed within the Quaternary alluvium (Shackley 1988). Evidently, there were two eruptive phases with crystalline histories different enough to produce slightly different chemistry.
To University of Sheffields WebElements and a discussion of the
periodic table of elements
![]()
Formation of a Glass
Two factors control whether a magma (melt) will form a glass, the rate of cooling and its viscosity, which is determined by its chemical composition. While it is theoretically possible to form a glass from most lavas, the presence of aluminum and silicon oxides in rhyolite greatly facilitates the process (Cann 1983; Cox et al. 1979). The high proportion of silicon (Si) [up to 78 wt. percent as SiO2] and aluminum (Al) [up to 15 wt. percent as Al2O3] in rhyolite melts is conducive to glass formation given sufficiently rapid cooling. Silicon and aluminum bond easily with oxygen in high temperature melts forming SiO2 and Al2O3, the glass formers. These oxides are responsible for the high viscosity of rhyolite lavas (Cox et al. 1979). Because of this high viscosity, rhyolite lavas frequently form dome structures rather than flows (like low viscosity basalt). Mount Elden and RS Hill in the San Francisco Volcanic Field, and many of the lesser known domes at Los Vidrios are classic examples of rhyolite dome structures (Nations and Stump 1981).
Classification scheme for volcanic rocks based on alkali element and silica content. Classification scheme of Cox, Bell, and Pankhurst (1980). Data for Hawaiian volcanoes is from Peterson and Moore (1987).
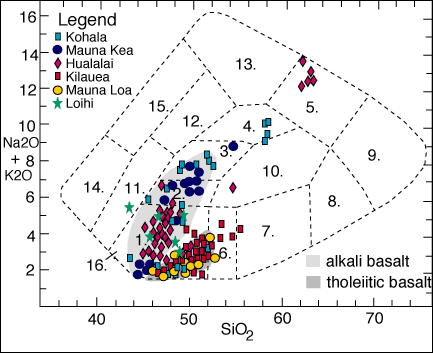
1. Basalt 2. Hawaiites 3. Mugearites 4. Benmoreites 5. Trachytes 6. Basaltic andesite
7. Andesites 8. Dacites 9. Rhyolites(incl. obsidian) 10. Trachyandesites 11. Basanites and Tephrites
12. Phonolitic tephrites 13. Phonolites 14. Nephelinites 15. Phonolitic nephelinites 16. Picrite
As rhyolite is extruded, super-cooling (quenching) creates a glass (obsidian). If there is sufficient H2O in the melt (>2%), perlitic and/or vitrophyric fabric may form in the glass. The resultant perlite or pitchstone is useless for the production of flaked stone tools since the rock exhibits preferred planes of cleavage. However, in some lavas, not all the glass will be hydrated and small remnants may occur called marekanites or "Apache Tears", that have not absorbed water (i.e. Burro Creek, Devil Peak, Los Vidrios, Sauceda Mts., Superior). Unfortunately for flaked tool production, most liquids rich in glass formers are also rich in water and other volatiles. Usually, eruption forms a froth creating pumice or vitrophyres, or is explosive and forms tuffs. This was a very common process in the Tertiary in Arizona. Most glass that is technically obsidian, but useless for tool production was extruded as vitrophyre or tuff, or subsequently hydrated to form perlite (see Shackley 1988). In order to produce aphyric, vitreous obsidian, the melt must have contained either a very low water content, or it must have been degassed in some way before eruption (Cann 1983; Cox et al. 1979).
Glass itself is not a very stable material. At low temperatures, a mixture of crystals is always more stable than a glass, and glasses will crystallize spontaneously if the atoms present have an opportunity to diffuse through the glass and become ordered (Cann 1983). This can occur if the glass is reheated, or by hot water percolating through the glass dissolving and reprecipitating atoms and compounds. This process can create vitrophyric fabric and the chalcedony geodes and strata commonly found associated with rhyolite structures in the Southwest.
Glass is also metastable at room temperature and pressure, and reacts with water and atmosphere primarily by solution, hydration, and clay alteration (Cerling et al. 1985; Friedman and Smith 1966). This process is at the basis of obsidian hydration dating studies, although the specific rate for a given source can be very complex to compute (Friedman and Long 1976; Michels et al. 1983).
![]()
This page maintained by Steve Shackley (shackley@berkeley.edu).
Copyright © 2008 M. Steven Shackley. All rights reserved.
Revised: Wednesday, 19 August 2015
![]() Back to
the SW obsidian source page
Back to
the SW obsidian source page
![]()